Arsenol
- 類別:
- Pharmaceutical
- What is Arsenic Trioxide?
- Arsenol: Rediscovery of an Oral Formulation
- Milestones
- Humanitarian Prespectives
- Publications
- Contact Us
What is Arsenic Trioxide?
Arsenic is a member of group Va of the periodic table. Arsenic trioxide (As2O3) is a white or transparent solid in the form of crystalline powder that resembles sugar. It has no odor or taste. It has low solubility in water; however, it dissolves readily in dilute hydrochloric solutions. It forms whenever elemental metallic arsenic is heated to high temperatures or burned. Arsenic trioxide can be absorbed by the digestive system and can cause acute poisoning when overdosed.
Despite the well known toxicity of arsenic, arsenic trioxide has been used medicinally for over 2000 years. Ancient Chinese used arsenic, also known as ‘‘Pi Shuang’’ in traditional Chinese medicine, to treat cancer and other conditions. The use of arsenic resurged when a medical group in Harbin, China, found that a crude intravenous preparation of arsenic trioxide, named Ailing-1, induced a high remission rate in acute promyelocytic leukemia (APL). The results were confirmed in a collaborative project between Harbin and Shanghai. This rediscovery of the therapeutic efficacy of arsenic in leukemia was an important milestone in the treatment of APL.
Intravenous arsenic trioxide is currently used to treat relapsed APL patients in the U.S. and the rest of the world except Hong Kong, where oral arsenic is used. Clinical studies have shown that complete remission rates between 78%-100% are obtained with intravenous arsenic trioxide treatment. However, there are many down sides associated with the intravenous formulation, including severe cardiac toxicity. This is because intravenous arsenic delays cardiac repolarisation by blocking repolarisation channels and prolongs the action potential duration in cardiac muscle. The cost of intravenous arsenic trioxide is inordinately high (about US$50,000 dollars a month), which makes this life-saving medication out of reach of leukemia patients in developing countries. Furthermore, there are also high hospitalization costs associated with intravenous arsenic trioxide.
Arsenol: Rediscovery of an Oral Formulation

Oral arsenic trioxide was first used in the Department of Medicine, Queen Mary Hospital (QMH), as a treatment of leukemia in the late 1940s and early 1950s. However, newer drugs phased out oral arsenic trioxide, and its use was forgotten for nearly half a century. In 1998, a small team of medical researchers at the Department of Medicine, Queen Mary Hospital, began investigating the use of oral arsenic in the treatment of blood cancers, based on the successful use of intravenous arsenic trioxide in the treatment of acute promyelocytic leukaemia. To determine the dosage and safety of oral arsenic, the QMH team initially turned to archival medical records retrieved from the Hong Kong Medical Museum. This was followed by two years of research into the methods of preparation of the oral formulation.
Since 2000, the team successfully prepared an oral formulation of arsenic that was tested in a clinical study in blood cancer treatment. With its demonstrated clinical efficacy, a series of patents has been granted including US, China, UK, France, Germany and Switzerland.
Milestones
ArsenolTM is also a drug developed entirely in Hong Kong and is poised to attain global status as a prescription medication. From 2018 to 2023, more than a hundred leukaemia patients in Hong Kong have been treated. After 28 months in peripheral blood test, all patients achieved complete remission and the 3-year overall survival rate achieved 97%. Oral arsenic trioxide gives a bioactivity similar to the IV formulation, but has lower peak plasma arsenic concentrations. Oral formulation improves the cardiac safety of arsenic trioxide, because of lower peak plasma arsenic concentrations. Oral arsenic trioxide is also convenient and safe for outpatients. Patients can take oral arsenic at home, rendering long-term therapy feasible and a massive saving in costs.
With oral arsenic (ArsenolTM) protected by intellectual property rights, Versitech is working on its global commercialization. A pre-IND meeting was held on 7 October 2024 that was attended by the lead researcher on acute promyelocytic leukaemia from HKU. The U.S. FDA officially announced Orphan Drug Designation for oral-ATO on 19 November 2024. This will be followed by a global registrational studies at HKU and major institutions in the U.S.A. and Europe in 2025 targeting NDA approval in 2027-2028.
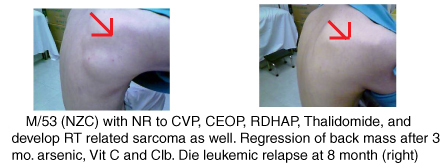
Humanitarian Prespectives
The University of Hong Kong is investigating the feasibility of making oral arsenic available on a compassionate basis to patients in developing countries, who face immense difficulties with drug costs, and the provision of in-patient hospital facilities and laboratory tests.
Arsenic trioxide is available as an intravenous drug in the US. The cost is about 50,000 US dollars (400,000 HK dollars) a month. The prohibitive cost makes this potentially life-saving medication out of reach of leukaemia patients in developing countries. Oral arsenic is set to replace intravenous arsenic trioxide as the standard formulation. Oral arsenic is very safe, and can be prescribed in the outpatient setting, obviating many of the medical problems in developing countries. A compassionate programme will save the lives of numerous underprivileged patients.
Publications
-
Pharmacokinetics
- Gill H, Yim R, Lee HKK, Mak V, Lin SY, Kho B, et al. Long-term outcome of relapsed acute promyelocytic leukemia treated with oral arsenic trioxide-based reinduction and maintenance regimens: A 15-year prospective study. Cancer. 2018;124(11):2316-26.
- Gill HS, Yim R, Kumana CR, Tse E, Kwong YL. Oral arsenic trioxide, all-trans retinoic acid, and ascorbic acid maintenance after first complete remission in acute promyelocytic leukemia: Long-term results and unique prognostic indicators. Cancer. 2020. 126(14): 3244-3254.
- Gill H, Raghupathy R, Lee CYY, Yung Y, Chu HT, Ni MY, et al. Acute promyelocytic leukaemia: population-based study of epidemiology and outcome with ATRA and oral-ATO from 1991 to 2021. BMC Cancer. 2023;23(1):141.
- Gill H, Yim R, Chin L, Lee P, Li V, Au L, et al. An Entirely Oral Regimen of Oral-Arsenic Trioxide/All-Trans Retinoic Acid/Ascorbic Acid in Newly-Diagnosed Acute Promyelocytic Leukaemia (APL): Updated Results of an Ongoing Multicentre Trial. Blood. 2023 Nov 28;142:157.
- Harinder Gill (12 Aug 2024): Chemotherapy-free approaches to newly-diagnosed acute promyelocytic leukaemia: is oral-arsenic trioxide/all-trans retinoic acid/ascorbic acid the answer?, Expert Review of Hematology, DOI: 10.1080/17474086.2024.2391098
-
Clinical & Safety Data
- W. Y. Au, C. S. Chim, A. K. W. Lie, R. Liang and Y. L. Kwong, Combined arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukemia recurring from previous relapses successfully treated using arsenic trioxide, British Journal of Haematology 2002. 117(1): 130-2.
- W. Y. Au, A. K. W. Lie, C. S. Chim, R. Liang, S. K. Ma, C. H. Chan, Y. K. Mak, Y. T. Chen, C. C. So, Y. M. Yeung, S. F. Yip, L. G. Wong, J. C. Chan, S. Y. Liu and Y. L. Kwong, Annals of Oncology 2003. 14(5): 752-7.
- Wing-Yan Au, Cyrus R. Kumana, Maybelle Kou, Raymond Mak, Godfrey C. F. Chan, Ching-Wan Lam and Yok-Lam Kwong, Oral arsenic trioxide in the treatment of relapsed acute promyelocytic leukemia, Blood 2003. 102(1): 407-8.
- Wing Y. Au, Chor Sang Chim, Albert Kwok Wai Lie, Annie Pang and Yok Lam Kwong, Real-time quantification of multidrug resistance-1 gene expression in relapsed acute promyelocytic leukemia treated with arsenic trioxide, Haematologica 2002. 87(10): 1109-11.
- Wing-Yan Au, Chi-Leung Liu, Sidney Tam, Bonnie M. W. Fong, Tony W. Shek, Chee-Kin Hui and Yok-Lam Kwong, Oral arsenic trioxide therapy for acute promyelocytic leukemia before and after liver transplantation for hepatitis B virus-related liver failure, Ann Hematol 2007. 86(10): 771-2.
- Chung-Wah Siu, Wing-Yan Au, Cindy Yung, Cyrus R. Kumana, Chu-Pak Lau, Yok-Lam Kwong and Hung-Fat Tse, Effects of oral arsenic trioxide therapy on QT intervals in patients with acute promyelocytic leukemia: implications for long-term cardiac safety, Blood 2006. 108(1): 103-6.
- Au WY, Li CK, Lee V, et al. Oral arsenic trioxide for relapsed acute promyelocytic leukemia in pediatric patients. Pediatr Blood Cancer. 2012;58(4):630–632. doi: 10.1002/pbc.2330614
- Burnett AK, Russell NH, Hills RK, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(13):1295–1305. doi: 10.1016/S1470-2045(15) 00193-X
- Gill H, Kumana CR, Yim R, Hwang YY, Chan TSY, Yip SF, et al. Oral arsenic trioxide incorporation into frontline treatment with all-trans retinoic acid and chemotherapy in newly diagnosed acute promyelocytic leukemia: A 5-year prospective study. Cancer. 2019;125(17):3001-12.
- Kumana CR, Au WY, Lee NS, et al. Systemic availability of arsenic from oral arsenic-trioxide used to treat patients with hematological malignancies. Eur J Clin Pharmacol. 2002;58(8):521–526. doi: 10. 1007/s00228-002-0514-x
- Gill H, Yim R, Lee HKK, et al. Long-term outcome of relapsed acute promyelocytic leukemia treated with oral arsenic trioxide-based reinduction and maintenance regimens: a 15-year prospective study. Cancer. 2018;124(11):2316–2326. doi: 10.1002/cncr.31327
- Gill HS, Yim R, Kumana CR, et al. Oral arsenic trioxide, all-trans retinoic acid, and ascorbic acid maintenance after first complete remission in acute promyelocytic leukemia: long-term results and unique prognostic indicators. Cancer. 2020;126(14):3244–3254. doi: 10.1002/cncr.32937(2020)
- Gill H, Yung Y, Chu HT, Au WY, Yip PK, Lee E, et al. Characteristics and predictors of early hospital deaths in newly diagnosed APL: a 13-year population-wide study. Blood Adv. 2021;5(14):2829-38.
Contact Us
| Address: | Versitech Limited 15/F, K. K. Leung Building The University of Hong Kong, Pokfulam Hong Kong |
| Email: | info@versitech.hku.hk |
| Contact telephone: | (852) 3917 0111 |