Broadly Neutralizing Antibodies & Robust Cellular Immunity Elicited by DNA Motif
- 領域
- Vaccine
- Reference No.
- IP00508
Key Problem and Market Opportunity
- HIV-1 has a high mutation rate which makes developing a vaccine against it a challenge
- Eliciting broadly reactive neutralizing antibodies (bnAbs) against HIV-1 remains elusive
- According to BCC Research, the global market for DNA vaccines is expected to be valued at USD $2.7 billion in 2019. Global sales for HIV DNA vaccines are forecasted to be USD $54.7 million
- The US and Europe are major geographical players in the vaccine market. In Asia, China is the largest vaccine market with high market growth due to increased government funding and demand
Key Advantages of the Technology
The V3 loop is a semi-conserved region of the gp120 envelope protein of HIV which is structurally constrained by its requisite participation in virus infectivity. Recent research suggested that the glycosylated V3 loop may be the key to elicit neutralizing antibodies.
This invention provides a DNA vaccination design to induce bnAbs against HIV-1 (DNA motif immunization directed to V3 loop of gp120 and polysaccharide epitopes). Meanwhile, the said DNA vaccine also induces long term cellular immunity through PD1 signaling pathway.
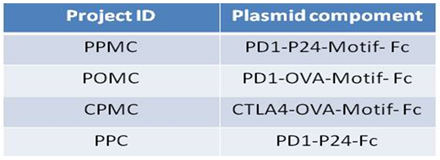
DNA motif vaccine design:
- GPG DNA motif (V3 loop of gp120)
- Glyco-DNA motif (polysaccharide epitopes)
Major effects against HIV:
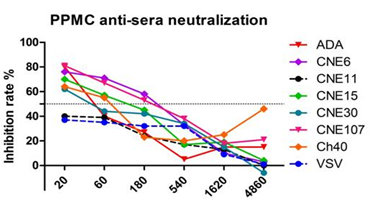
- Humoral immunity: to induce bnAbs against HIV-1 comprising of a DNA motif
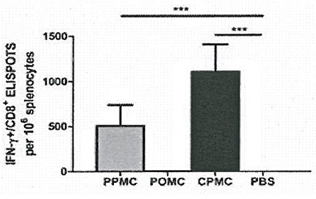
- Cellular immunity: to induce cellular immunity using a DNA motif through PD1 signaling pathway
Potential Product and Services
- DNA vaccine against HIV infection and novel immunity strategy
Development Status
Stage of Development
- Completed proof of concept of DNA motif immunization and prototype available
Patents
- US Patent No. 10,245,313 issued on 2 Apr 2019
- EP Patent Application No. 15852067.6
- China Patent Application No. 201580057749.2
IP Status
- Patented
- Patent application submitted