A Live Attenuated Influenza Virus Vaccine Offering Universal Protection
- Field
- Vaccine
- Reference No.
- IP00889
Background
- According to Allied Market Research, the global influenza vaccine market size was valued at $3.96 billion in 2018, and is projected to reach $6.20 billion by 2026, registering a CAGR of 5.9% from 2019 to 2026.
- Seasonal influenza viruses change constantly, which further leads to modifications in influenza vaccines to combat season-specific viral strains.
- Influenza A viruses have multiple HA subtypes that are antigenically diverse. Classical influenza virus vaccines are subtype specific, and they cannot induce satisfactory heterosubtypic immunity against multiple influenza virus subtypes.
- Therefore, there is a need to develop an universal and robust vaccine that can provide protection against flu variants.
Technology Overview
- Anti-galactose-1,3-galactose (anti-Gal) antibody is naturally expressed at a high level in humans. Here, we designed a live attenuated influenza virus vaccine (NAGT mutant) that can generate-Gal epitopes in infected cells in order to facilitate opsonization of infected cells, thereby enhancing vaccine-induced immune responses.
- The animals expressing anti-Gal antibody in vivo can be fully protected by our vaccine against a lethal H1, H5 or H3 virus challenge. HKU’s work demonstrates a new strategy for using a single influenza virus strain to induce broadly cross-reactive immune responses against different influenza virus subtypes.
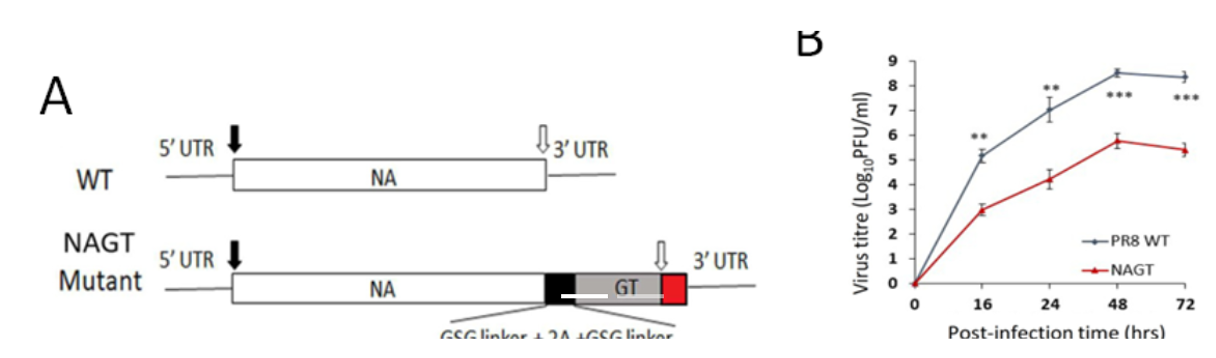
- Figure 1. Characterization of the NAGT mutant on H1N1 backbone
(A) WT and mutated NA segments in the cRNA sense strand. A mouse -1,3-GT gene ORF was introduced immediately before the stop codon of the NA ORF (open arrow). (B) Replication kinetics of NAGT mutant in MDCK cells
- Figure 2. The NAGT mutant can protect mice against a lethal homologous and a lethal heterosubtypic challenge
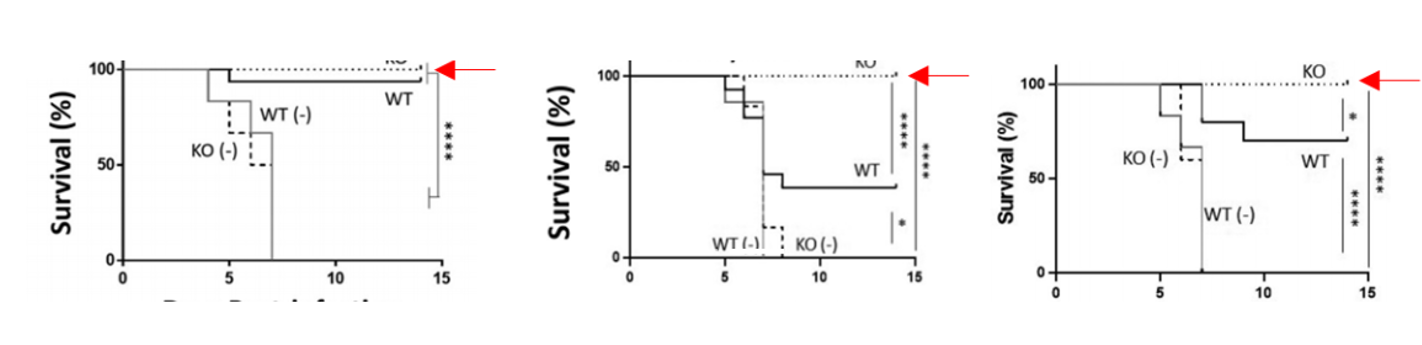
Further Details
Journal publication: mBio 2020 Mar 3;11(2):e00027-20. DOI: 10.1128/mBio.00027-20
Benefits & Applications
Benefits
- Novel, first live-attenuated vaccine to exploit anti-galactose-alpha 1,3-galactose antibodies
- Potential to provide heterosubtypic protection, a universal influenza A vaccine
- Highly robust antibody and T cell responses upon challenge
Applications
- Influenza vaccine
Patents
PCT application No. PCT/CN2021/071079 filed 11 Jan 2021
IP Status
- Patent application submitted