A New Class of Metallo-β-lactamases inhibitors Against Antibacterial Resistance
- Field
- Therapeutic Chemicals
- Reference No.
- IP00603
Background
- Due to widespread antibiotics use, bacterial resistance is increasing and now become a serious threat. Each year, over 2 million people are estimated to be infected by antibiotic-resistant bacteria, where at least 23,000 people died each year as a consequence.
- β-lactam antibiotics are a class of broad-spectrum antibiotics used widely. The most prominent resistant mechanisms are the expression of β-lactamase enzymes, for which no clinically available wide-spectrum inhibitors are available.
- According to Grand View Research, the antibiotics market is expected to reach USD 57.0 billion by 2024.
Technology Overview
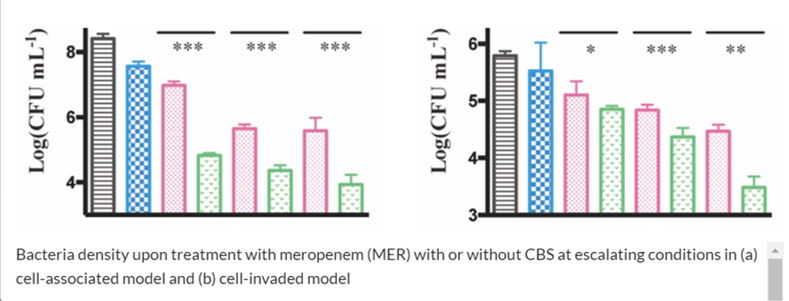
- Figure 1. In vitro test (Bacteria density upon treatment)
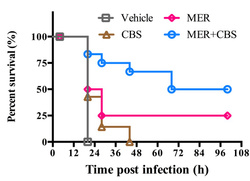
- Figure 2. In vivo test (Bacteria challenge in mice)
- This invention disclosed the use of some bismuth (III) compounds as metallo-β-lactamases (MBLs) inhibitors. They can be administered in combination with β-lactam antibiotics to treat MBL-producing bacterial infection.
- In vitro and in vivo data showed that such bismuth compounds were able to inhibit the MBLs, rendering the effectiveness of the beta-lactam antibiotics (including amoxicillin, ampicillin and meropenem) against multiple resistant strains.
- The bismuth compounds include but not limited to Colloidal bismuth subcitriate (CBS), Bismuth subsalicylate (BSS), Bismuth subgallate (BSG) and Ranitidine bismuth citriate (RBC) which are FDA-approved bismuth drugs and are safe to be used in humans.
Benefits & Applications
Benefits
This invention offers a solution to treat infections caused by bacteria resistant to beta‑lactam antibiotics.
Applications
Pharmaceutical composition for prevention or treatment of infection caused by metallo-β-lactamases (MBLs) producing bacteria.
Patents
- US Application No. 15/278,916
- PCT Application No. PCT/CN2017/102077
IP Status
- Patent application submitted
Seeking
- Development partner
- Commercial partner
- Licensing