Mobile SARS-Cov-2 Detection/COVID-19 Diagnostic Assay
- Field
- Diagnostics
- Patent
- IP00946
Key Problem and Market Opportunity
- To control the COVID-19 pandemic and prevent its resurgence in areas preparing for a return of economic activities, a method for a rapid, simple, and inexpensive diagnosis and mass screening is urgently needed
- Currently, the gold standard for SARS-CoV-2 detection is based on qRT-PCR
- SARS-CoV-2 detection by qRT-PCR requires expensive qPCR machines and regents. Thus the cost is high, and the assay is not deploy-able on mobile units
- Hence, a low cost, simple and mobile COVID-19 diagnostic assay is needed
Key advantages of the technology
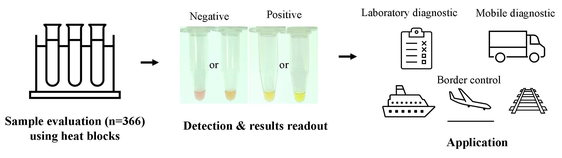
- A one-step colorimetric reverse-transcriptional loop-mediated isothermal amplification assay (COVID-19-LAMP) for detection of SARS-CoV-2
- The sensitivity and specificity of are 98.21% and 100% respectively
- The assay involves simple equipment and techniques and is low cost, without the need for expensive qPCR machines. The result, indicated by color change, is easily interpreted by naked eyes
- can detect SARS-CoV-2 RNA with a detection limit of 42 copies/reaction
- can be useful for rapid deployment as mobile diagnostic units to resource-limiting areas for point-of-care diagnosis, and for unlimited high-throughput mass screening at borders to reduce cross-regional transmission
Potential Product and Services
- COVID-19-LAMP Rapid Diagnostic Kit
- Simple, rapid, low cost COVID-19 diagnostic services in medical laboratories, mobile diagnostic units for border control, lockdown areas, remote areas, developing countries etc
Development stage and IP strength
- US Provisional Patent application: 63/024,887
- Journal publication: https://www.mdpi.com/1422-0067/21/15/5380
- To control the COVID-19 pandemic and prevent its resurgence in areas preparing for a return of economic activities, a method for a rapid, simple, and inexpensive diagnosis and mass screening is urgently needed
- Verified with more than 380 clinical samples.