PD1-Based TWIST1 Cancer Vaccine
- Field
- Therapeutic Biologics
- Reference No.
- IP00870
Key Problem and Market Opportunity
Background
-
Checkpoint immunotherapy is a major breakthrough for cancer treatment, yet its efficacy is often limited against many types of malignancies.
-
In order to enhance the efficacy of existing immunotherapy, it may be necessary to elicit antitumor responses through active vaccination.
-
Cancer vaccines involve boosting and proper activation of patients’ own immune surveillance. The global cancer vaccines market was valued at $4,188 million in 2019, and is projected to reach $7,303 million by 2027, registering a CAGR of 12.6% from 2020 to 2027.
Technology Overview
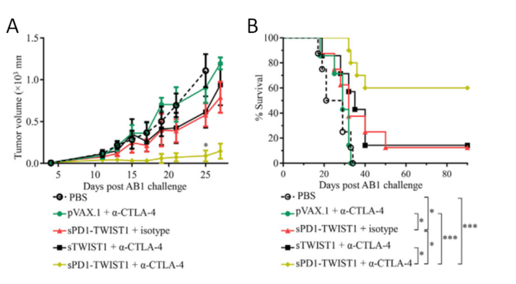
TWIST1, the basic helix-loop-helix transcription factor, is associated with human mesothelioma tumorigenesis and required for the invasion and metastasis of mesothelioma in the immune-competent murine AB1 model. The programmed cell death protein 1 (PD1)- based vaccination provided prophylactic control by inducing long-lasting TWIST1-specific T cell responses against both subcutaneous and metastatic mesothelioma lethal challenges. Furthermore, while CTLA-4 blockade alone did not show any immunotherapeutic efficacy against established mesothelioma, its combination with PD1-based vaccination resulted in 60% complete remission.
Figure 1. Checkpoint Modulation Enhances the Antitumor Activity of sPD1-TWIST1 Vaccination for Curing Established Mesothelioma
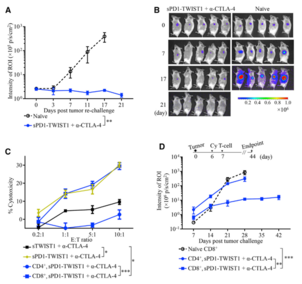
Figure 2. Combination Therapy Induced Durable T Cell Immunity Responsive to an Immunodominant TWIST1 Epitope
Further Details
Mol Ther Oncolytics. 2020 Mar 27; 16: 302–317. doi: 10.1016/j.omto.2020.01.009
Key Advantages of the Technology
- Eliciting long-term memory CD8+ T responses against tumors.
- Demonstrated efficacy in treating mesothelioma and breast cancer
Potential Product and Services
Cancer vaccines that enhance immunotherapy against tumors
Development Status
Patents
- US Provisional Application No. 62/978,911
IP Status
- Patent application submitted